
Geeta Nayyar, MD, MBA, discusses how hydroxychloroquine and chloroquine affect patients with arthritis and how it differs for those with coronavirus.

Geeta Nayyar, MD, MBA, discusses how hydroxychloroquine and chloroquine affect patients with arthritis and how it differs for those with coronavirus.
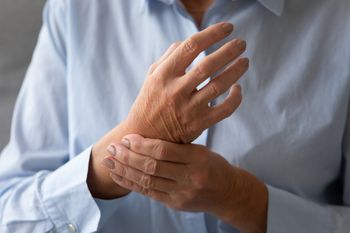
The American College of Rheumatology recommends treating rheumatoid arthritis early in order to increase the chances of achieving and sustaining remission. Take our clinical quiz to learn more.
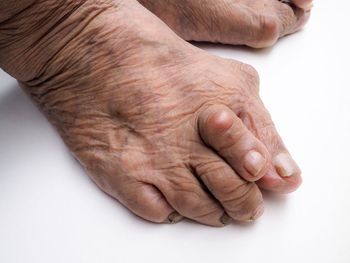
Colchicine, a first-line treatment option for acute gout, is associated with an increased risk of adverse events, such as diarrhea and other gastrointestinal symptoms, but not serious adverse events, finds a newly published systematic review and meta-analysis.

The device may allow for more natural motion and increased wrist durability over traditional implants.
The use of abatacept did not lead to significant improvements in the disease status of patients with primary Sjögren's syndrome and in fact, it was essentially no better than placebo treatment, shows the first randomized, double-blind trial on abatacept for Sjögren’s.
Despite the publication of previous clinical trials that showed plasma exchange could possibly be a viable treatment for patients with severe ANCA-associated vasculitis, a new study recently published in the New England Journal of Medicine shows the treatment, in combination with standard therapy, didn't improve outcomes for patients or lower fatality rates or slow the progression to end-stage kidney disease.
On Tuesday, the World Health Organization announced it is recommending that people with COVID-19 symptoms avoid taking the anti-inflammatory drug ibuprofen. U.S. officials and physicians responded with skepticism and today, WHO reversed its decision.

Dr. Philip Robinson, a rheumatologist in Brisbane, Australia, is in the process of creating an international registry that will include information about COVID-19 specifically for patients with rheumatic disease, in particular, those receiving immunosuppressive therapies.

A study compares the risks associated with an anterior surgical approach to a lateral or posterior approach.
A new study published in the February 5 issue of Annals of the Rheumatic Diseases finds that allopurinol and febuxostat are associated with about the same risk of hypersensitivity reactions, as compared to gout patients treated with colchicine. The risk is even higher for women and diabetes patients, the authors wrote.

Sarilumab is typically used for patients with rheumatoid arthritis but could be useful for patients with novel coronavirus.
March is Women's History Month and in today's issue of the column Awkward Conversations, Dr. Kim Gorgens and Sophia Barnes focus on challenges women in science face. They also offer some key resources you may want on your radar.

To celebrate Women’s History Month, we’re featuring important female figures in rheumatology. In this Q&A with Silvia Ross, M.D., F.A.C.R., managing partner of Triangle Arthritis & Rheumatology Associates, Raleigh, North Carolina, and co-chair of Global Affairs for the Association of Women in Rheumatology, we focus on her work history, her accomplishments, and her future.
The risk of dying from a cardiovascular event can increase by 50 percent for rheumatoid arthritis patients with low serum folate levels, report researchers in JAMA Open Network.
The U.S. Food and Drug Administration has approved the first-ever treatment for chronic fibrosing interstitial lung disease (ILD), a condition that can affect rheumatoid arthritis patients. The treatment is designed for patients with a form of the disease that progressively worsens over time.

Digital health tools can improve physical function and serve as an alternative treatment option for patients with certain chronic conditions.

The findings can lead to new policies, procedures, and programs being implemented at health systems.
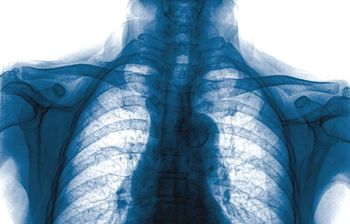
Respiratory viral infections can be a novel environmental risk factor for the development of rheumatoid arthritis, find Korean researchers writing in Arthritis Research and Therapy.
A two-pronged vaccination approach performs better than a single pneumococcal vaccination in patients receiving conventional DMARDs or abatacept-treated patients, but not in patients receiving rituximab, say researchers writing in Arthritis Research & Therapy.

Providers who care for patients with asthma or COPD need to be aware of the increased risk of rheumatoid arthritis.
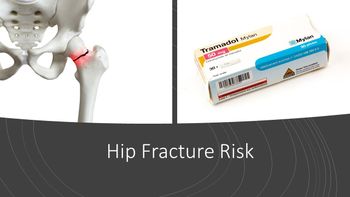
The use of tramadol in older patients is associated with a higher risk of hip fracture compared with the use of codeine or commonly used nonsteroidal anti-inflammatory drugs (NSAIDs), say researchers recently writing in in the Journal of Bone and Mineral Research.
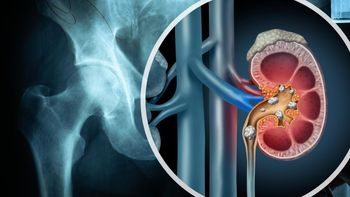
Hip replacements may not be the best choice for acute kidney injury patients, shows a new study that highlights above normal rates of death, longer hospital stays, revisions, transfusions, implant problems, above norm hospital charges; and, discharge to rehab hospitals.

Tocilizumab might be a useful adjunctive therapeutic option for children with juvenile idiopathic arthritis-associated uveitis that doesn't respond to TNFi treatment, say researchers recently writing in The Lancet Rheumatology.

The GSK-branded Advil will be available as a dual-action pain reliever later this year.

The U.S. Food and Drug Administration has approved a label update for secukinumab (Cosentyx, Novartis) to include the option for up-titration to a 300 mg dose for adults with active ankylosing spondylitis.

Adding methotrexate to adalimumab (Humira, AbbVie) in axial spondyloarthritis was associated with reduced immunogenicity, and prolonged co-medication was associated with long-term maintenance of adalimumab, say researchers recently writing in RMD Open: Rheumatic & Musculoskeletal Diseases.
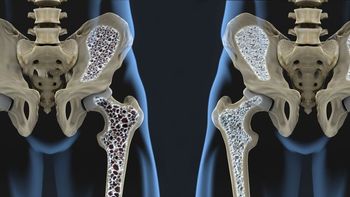
Bone mineral density was preserved over a three-year period in patients with rheumatoid arthritis treated with biological/targeted synthetic disease-modifying antirheumatic drugs (DMARDS) but declined in those who received only conventional-synthetic DMARDs, say researchers recently writing in Rheumatology.

Research from the University of Texas examines how serum folate levels above or below a certain level were linked to an increase in cardiovascular mortality.

The FDA has expanded the indication of the over-the-counter pain reliever ActiPatch (BioElectronics Corp.) to include all musculoskeletal pain. Learn more about this and other recent drug approvals in this news brief.

The U.S. Food and Drug Administration has approved the use of an intravenous form of the pain reliever meloxicam (Anjeso, Baudax Bio) for the relief of moderate to severe pain.