A recent study identified the prevalence of asymptomatic pulmonary involvement in patients with newly diagnosed RA or PsA, highlighting the need to reevaluate screening protocols.

A recent study identified the prevalence of asymptomatic pulmonary involvement in patients with newly diagnosed RA or PsA, highlighting the need to reevaluate screening protocols.

The exploratory MIRROR trial found pegloticase with methotrexate improved clinical assessment and quality of life outcomes in uncontrolled gout.

Cell therapy, particularly CAR-T, is expanding into autoimmune diseases like lupus and multiple sclerosis. While promising, safety, efficacy, and broad applicability remain uncertain.

This year in review spotlights top rheumatology FDA news from 2024 and new research out of the ACR 2024 Convergence.

After 16 weeks of treatment, a significantly greater proportion of deucravacitinib-treated patients achieved ACR20 response.

The COSMOS trial showed guselkumab every 8 weeks improved PsA outcomes through week 48, including in patients with inadequate responses to TNF inhibitors.
A prospective study also found that the presence of psoriasis and dactylitis at disease onset were the strongest predictors for the development of PsA.

A study found the association between sedentary behavior and gout remained strong even when adjusting for physical activity.

These findings, from an observational study, should be confirmed in a randomized, controlled trial.

The FDA has accepted the NDA for TNX-102 SL, a non-opioid, centrally acting analgesic, for the bedtime treatment of fibromyalgia; the PDUFA date still needs to be assigned.

The rheumatology month in review emphasizes new data from ACR 2024 in lupus, gout, and fibromyalgia.

Women who gave birth after infertility without receiving fertility treatments had an elevated risk of developing SARDs, such as lupus.

CDC, NIH, and AMA recommend PEMs to be at a 6th-to-8th-grade reading level, while assessed materials were readable at high school or higher levels.

CT-P41 had similar rates of bone mineral density increases and adverse events in data presented at the ACR 2024 Convergence.

The GLEAM trial is currently enrolling patients to receive SC291, with data expected in 2025.

Ustekinumab-kfce is planned for launch in February 2025 according to a previous settlement and license agreement with Janssen.

Findings from PsABIOnd can help reassure clinicians of similar efficacy between treatment choices.
At the highest dose levels, mean plasma urate levels remained below 6.0 mg/dL for up to 12 weeks.

Patients in the anifrolumab arm also had lower rates of damage accrual.

The findings are especially important as differences in disease characteristics and outcomes between male and female patients become more clear.

Lowering serum urate level to less than 6 mg/dL was not linked to an elevated risk of severe or end-stage kidney disease progression in patients with gout.

This post-hoc analysis demonstrated an association between work productivity and stringent disease control criteria for those with psoriatic arthritis.

Hypovitaminosis D was more prevalent in patients with lupus nephritis compared to patients with systemic lupus erythematosus without kidney disease.
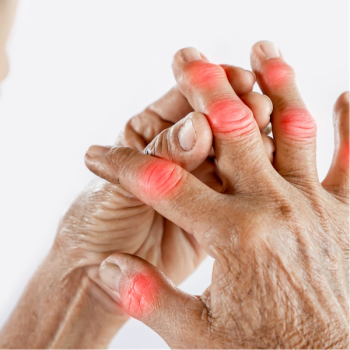
Clinically meaningful increases in patient-reported outcomes were identified after 3 doses of SEL-212 and strengthened after 3 additional doses.

These data, presented at ACR 2024, demonstrate that response was maintained at 2 years among those with psoriatic arthritis who responded to bimekizumab at Week 16.

The Urate-Lowering Therapy to Acute Treatment Ratio (ULTrA) was linked to fewer gout-related hospital admissions.

Infusion reaction rates and serum level lowering were similar between 120-minute and 60-minute infusion cohorts in the phase 4 AGILE study.

Clowse discussed findings from the phase 3 PHOENYCS GO trial presented at the 2024 ACR Convergence.

Data from 2 studies presented at ACR 2024 demonstrate the potential of AI to improve access to quality rheumatological assessments.