
The rheumatology month in review emphasizes new data presented at the 2025 ACR Convergence.

The rheumatology month in review emphasizes new data presented at the 2025 ACR Convergence.

New research at ACR 2025 highlights the link between cognitive impairment and disease activity in SLE.

Ianalumab significantly reduces Sjögren disease activity, meeting the primary outcome in recent phase 3 trials.

Mepolizumab shows significant real-world benefits in reducing end-organ dysfunction.

Switching to an IL-17 inhibitor after TNF inhibitor failure showed no superior benefits for axial spondyloarthritis.

TENS combined with physical therapy significantly alleviates fibromyalgia pain and fatigue.

A recent exploratory analysis reveals significant kidney B-cell depletion by the recently approved drug.

Telitacicept significantly alleviates Sjögren disease symptoms over 48 weeks, outperforming placebo with a favorable safety profile in a phase 3 trial.

In long-term follow-up of KEEPsAKE 1 and 2, risankizumab maintained joint, skin, pain, and axial responses in patients with PsA through 244 weeks.

Stay updated with the latest healthcare breakthroughs, including FDA approvals and colorectal cancer screening updates, in this week's essential news roundup.

View slated expert interviews and 6 clinical RA, PsA, and lupus trials to watch at ACR 2025.

The decision is supported by positive results from the phase 2 NOBILITY and phase 3 REGENCY studies.

However, a notable higher rate of treatment-related adverse events warrants caution.

Sonelokimab meets ACR50 primary endpoint in a phase 2 trial.

Stay updated with the latest healthcare breakthroughs, including FDA news, regulatory submissions, and evolocumab data for ASCVD, in this week's essential news roundup.

Q3 2025 saw a number of exciting approvals and data on promising therapies in development.

Q3 2025 saw several new first targeted therapies for rare and more individualized indications in a number of fields.

The rheumatology month in review emphasizes new approvals and the latest comparative research.

Mease discussed his hopes for the new approval's impact on the fibromyalgia field.

The approval makes guselkumab the first and only IL-23 inhibitor approved for these pediatric indications and was based on data from the phase 3 PROTOSTAR study.

Risankizumab shows significant and lasting improvements in psoriatic arthritis symptoms, addressing multiple disease domains and enhancing patient quality of life.

Upadacitinib reduced glucocorticoid doses and improved disease activity over 104 weeks.

Study data favored the placebo group, although differences were not clinically meaningful.
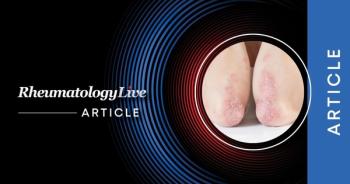
A new meta-analysis has contrasted the association between the 2 conditions against the general population, psoriasis, rheumatoid arthritis, and ankylosing spondylitis.

Nearly a third of patients with lupus in APPEAL reported falls, with disease activity, depressive symptoms, and medication use among key risk factors.

Compared with non-use, taking semaglutide or tirzepatide was associated with improved rheumatoid arthritis disease activity and cardiovascular risk.

The investigational therapy from Innovent Biologics, Inc. displayed significantly greater urate-lowering efficacy versus febuxostat, along with a favorable safety profile.



Combining golimumab with methotrexate reduced corticosteroid reliance and maintained efficacy in psoriatic arthritis.