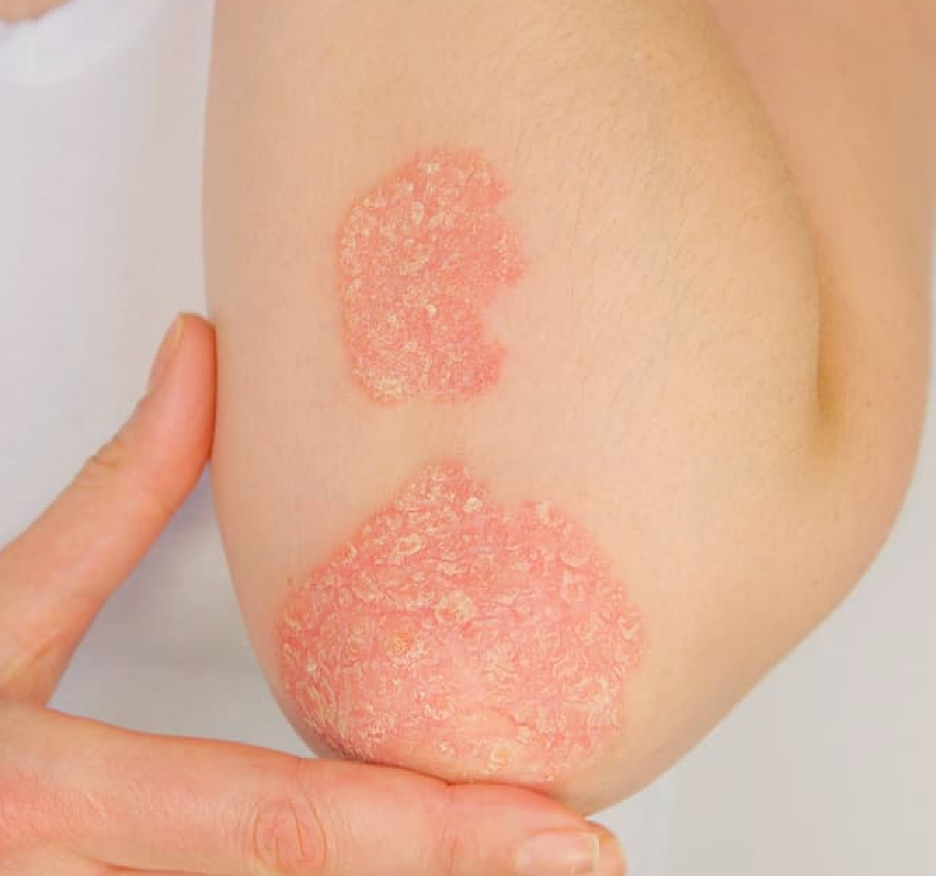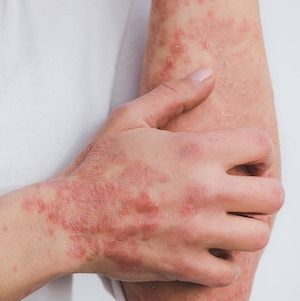
Psoriatic Arthritis
Latest News
Latest Videos

More News

These data on the success of twice-daily, 5 and 10 mg doses of tofacitinib may help inform clinicians in their practice as well as the health community in general.
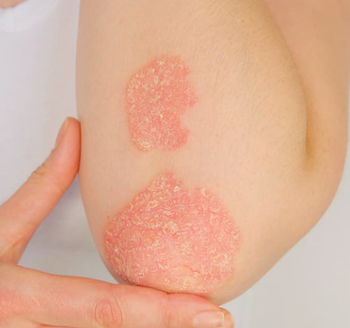
These findings on both remission and low disease activity suggest that some individuals in routine clinical practice may see benefits after extending treatment with etanercept past 12 weeks.

Patients with rheumatic disease treated with biosimilar SDZ ETN reported high treatment persistence at month 12 as well as comparable safety and efficacy.

Patients with inflammatory arthritis demonstrated significantly increased odds of depression and anxiety health care encounters and use of anxiolytics and antidepressants.

Results showed no differences regarding safety, although the biosimilar group had slightly more discontinuations due to inefficacy.

BLA submission was based on data from phase 3 clinical trials in which DMB-3115 showed no clinically meaningful differences compared with the reference product for the treatment of plaque psoriasis.

In this discussion with Dr. Mease at ACR 2023, he covers findings on an assessment tool known as the PsA-5Ts for patients with psoriatic arthritis.

This interview segment with Dr. Mease featured a discussion about data presented at ACR 2023 on guselkumab and patients with psoriatic arthritis.

This segment of Dr. Kivitz’s interview at ACR 2023 featured a discussion about future research on TAK-279 as well as general outlook on the field for psoriatic arthritis.

During this interview, Dr. Kivitz explained the background and key findings of his team’s research on TAK-279, a selective oral TYK2 inhibitor.

At the 7-month mark, multivariate analysis revealed patients with psoriasis aged ≥65 years reported a significantly better response to tildrakizumab.

Syndesmophyte development was 4 times more probable when patients had a clinical or radiographic description of axial involvement combined with elevated C-reactive protein.

The FDA has expanded the approval of abatacept (Orencia) for the treatment of active psoriatic arthritis in patients aged 2 years and older.

Results from a large observational retrospective cohort of ≥500 patients with PsA show relatively low drug persistence of apremilast, an oral PDE4 inhibitor.
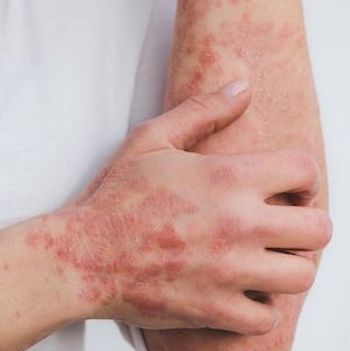
24-week results from the UPREAL show higher efficacy of upadacitinib among males with PsA, bio-naïve patients, and those with elevated baseline C-reactive protein.

Many studies support the finding that biologic therapy reduces the presence of psoriatic arthritis—or the risk of developing arthritis in psoriatic patients—but other research suggest the opposite.

A cross-sectional study of survey data shows more than 50% of psoriasis and over 60% of psoriatic arthritis patients have depressive symptoms, with results drawing a link between functional impairment and the likelihood of depressive symptoms.

While Black people are consistently underrepresented, White people are consistently overrepresented in inflammatory arthritis trials for rheumatoid arthritis, psoriatic arthritis, spondyloarthritis, and juvenile idiopathic arthritis.

Results from Mendelian randomization analyses revealed PsO and PsA did not increase the risk of basal cell carcinoma, cutaneous squamous cell carcinoma, or cutaneous melanoma.

Sex, age at disease onset, and psoriatic arthritis were associated with the greatest impact on major life-changing decisions in patients with psoriasis.
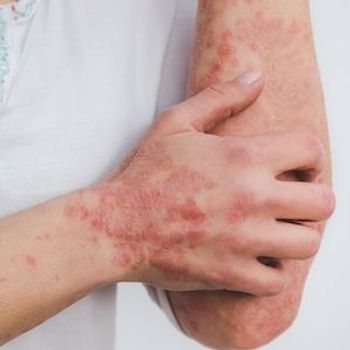
The real-world evidence study examined patients with PsA taking secukinumab and ixekizumab, highlighting 80% treatment persistence after 1 year.

Bimekizumab was found to be more cost-effective than most biologics and targeted synthetic disease-modifying antirheumatic drugs in a Swedish healthcare system-based model.

Results demonstrated a low persistence rate for apremilast among patients with PsA, highlighting a notable barrier to treatment success.

Achievement of minimal disease activity and remission were observed in approximately half of patients with psoriatic arthritis receiving upadacitinib.

The approval of the intravenous formulation of secukinumab allows healthcare providers to better tailor treatment to their patients’ unique needs.