
A significant number of patients with psoriatic arthritis fail to achieve adequate disease control, resulting in health-related quality of life (HRQOL) impairments.

A significant number of patients with psoriatic arthritis fail to achieve adequate disease control, resulting in health-related quality of life (HRQOL) impairments.

Dactylitis, a uniform swelling of an entire digit, occurs in up to half of patients with psoriatic arthritis and is sometimes the first manifestation of the condition.

“Evidence has accumulated on the effectiveness of physical activity to reduce disease-related symptoms such as pain and comorbidity risk in people with rheumatoid arthritis,” investigators stated.

The post hoc analyses evaluated health-related quality of life and efficacy across 4 subgroups treated with either ixekizumab or adalimumab.

Test-retest reliability for Health Assessment Questionnaire–Disability Index and 36-item Short Form Health Survey physical functioning domain in patients with psoriatic arthritis was judged to be good and reasonable, respectively.

Results show how patients with psoriatic arthritis prioritize their decisions when choosing medication.

The efficacy evaluation of golimumab is crucial for patients with psoriatic arthritis, particularly for treating skin manifestations and psoriasis symptoms.

Early diagnosis and treatment of psoriatic arthritis leads to better long-term outcomes. Despite this, most patients have significant diagnostic delays.

Investigators analyze the differences between SARS-CoV-2 testing and risk of infection.

Investigators found that patients who were not treated with biologic medications were at a significantly increased risk of developing psoriatic arthritis.

Alexis Ogdie-Beatty, MD, discusses the UPLIFT survey and UPLIFT Innovation Challenge, the unmet needs of patients with psoriatic arthritis, and the clinical significance of the survey’s results.

Compared with controls, patients with psoriatic arthritis presented more often with depression at baseline and patients with rheumatoid arthritis had a higher incidence of cardiovascular comorbidity after 3 years.

Philip J Mease, MD, discusses his study, “Guselkumab demonstrated an independent treatment effect in reducing fatigue after adjustment for clinical response—results from two phase 3 clinical trials of 1120 patients with active psoriatic arthritis.”

In this Q&A, corresponding author Josef S Smolen, MD, discussed the findings of his study determining the effectiveness of ustekinumab versus tumor necrosis factor inhibition in patients with psoriatic arthritis.

Researchers record more depressive symptoms in patients with psoriatic arthritis, which they believe incentivizes doctors to utilize screening methods that focus on the mental health of a patient.

Guselkumab, an interleukin-23p19-subunit inhibitor, led to clinically significant and sustained improvements in fatigue through 1 year in patients with active psoriatic arthritis.

Catch up on Rheumatology Network's most recent highlights in psoriatic arthritis news.

Researchers from France challenge previous reports of the IL-17 inhibitor treatment resulting in an increased risk of inflammatory bowel disease in patients with immune disorders like psoriasis.

Patients with psoriatic arthritis often feel limited in their knowledge on medications and treatments, which investigators said leads to a lack of treatment and increased risk of disability.

“This analysis provides information on when clinically meaningful improvements in pain may be expected in patients with psoriatic arthritis receiving tofacitinib or adalimumab, and how baseline pain severity may impact response to tofacitinib, which is of value in clinical practice,” stated investigators.
Research reveals microtrauma and swelling in the joints surrounding the thumb, conditions that are predominantly features in patients with psoriatic arthritis than any other arthritic diseases.

Christopher Ritchlin, MD, MPH, discusses the safety and efficacy of bimekizumab in patients with psoriatic arthritis, the BE ACTIVE study, the clinical significance of these results, and his plans for future research on this topic.

The US Food and Drug Administration (FDA) has delayed its decision on upadacitinib (RINVOQ) for the for the treatment of adults with active psoriatic arthritis and adults with active ankylosing spondylitis.

In patients with early inflammatory arthritis, a serum protein biomarker panel was proven effective in separating patients with psoriatic arthritis from those with rheumatoid arthritis.
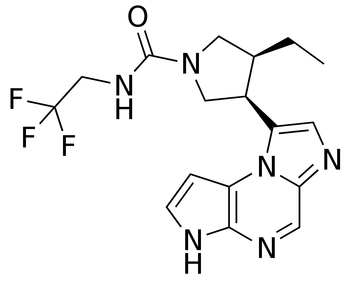
Upadacitinib (UPA) effectively treated patients with psoriatic arthritis (PsA) and axial involvement, a group that is historically more likely to have higher disease burden and more quality-of-life impairments than patients without axial involvement.